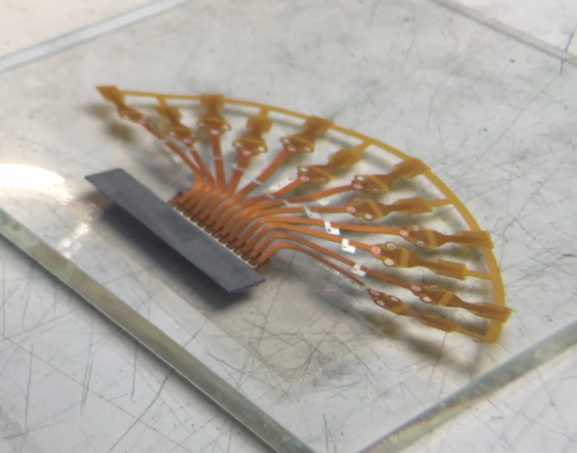
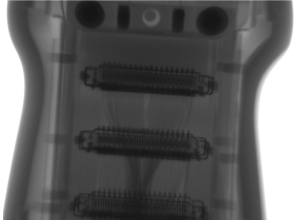
We’re often asked, “What are some of the most common failures with ultrasound probes?”. We can confidently say, “It depends”. We’re also asked, What’s the best method of field testing …
ISO 13485:2016 Certified Quality Management System
Did you know? Innovatus only performs repairs on devices for which we have a process documented in our ISO 13485:2016 certified Quality Management System. Commitment to Quality In the repair …
Uniquely Qualified to Repair Your Ultrasound Probes
Did you know? Innovatus Imaging is an FDA-registered medical device Original Equipment Manufacturer (OEM) as well as an Independent Service Organization (ISO). Innovatus manufactures ultrasound probes, ultrasound arrays, and ultrasound-related …
Invivo MRI Coil Solutions
Innovatus Imaging has comprehensive Invivo MRI coil solutions on almost 200 MRI coils manufactured by Invivo. Performance, function, and safety are fully assessed top-to-bottom, so there are no surprises later-on, …
What’s Inside Matters
If we’ve learned anything over the years, that applies to the Right to Repair issue that we now face relative to a repair, is WHAT’S INSIDE MATTERS. Back in 1991, …
Elements of Speed
No matter what’s going on in the world around us, turn-around time will always be a critical issue for maintaining uptime, client satisfaction, and patient care for healthcare facilities of …
Do, or do not. There is no try.
Seriously, anyone can repair just about anything, as long as you have some basic skills, a few tools, hand-eye coordination, a garage with electricity, and YouTube. But should they? There …
The Road Less Traveled
Years ago, our founders paved a new path for probe repair and manufacturing by developing technology and processes no one had heard of before, let alone applied. Many in the …
Processes, Procedures, and Work Instructions
by Matt Tomory, Vice President of Sales and Marketing When a company documents its Quality Management Systems (QMS), it must clearly define what it’s doing (Processes) or what its Procedures …
HTM’s Role in Ultrasound Accreditation
How Accreditation for Imaging Departments Affects Your Facility, Your Patients, and You The importance of being ISO 13485:2016 certified has been a popular topic in the medical device service industry …