If we’ve learned anything over the years, that applies to the Right to Repair issue that we now face relative to a repair, is WHAT’S INSIDE MATTERS. Back in 1991, Intel Corporation began placing “intel inside” stickers on PCs built with its processors so that consumers would know that their computer contained a genuine Intel microprocessor and not a knockoff, and possibly inferior, CPU. Skeptics claimed the general public did not care about the “ingredients” – only if the overall product appeared to perform.
Lately we’ve had many conversations with our clients and partners which include multiple OEMs, Independent Service Organizations, Asset Managers, E-retailers, and Healthcare Providers about the recent activity with the FDA regarding Right to Repair and the ever-present clarifications on the definitions of repair, servicing, re-manufacturing, and restoring. It has become very clear that the medical device repair market is rotating and de-commoditizing. While price is always a concern, more and more we are discussing processes, materials, verification, validation, Quality Management Systems, and other aspects of proper repairs which can demonstrate that a repair event has returned a medical device to perform as the OEM intended and is safe and effective versus just functioning again.
Think about that last statement for a moment: What is the difference between functioning and performing as the OEM intended?
At Innovatus, we are constantly trying to find ways to communicate to healthcare providers, and those that serve them, what we do and how we do it because what goes into a holistic repair is so critical to patient care, sustainability, long term value, and safety. We do not just fix medical devices – we return ultrasound probes and MRI coils, which are sophisticated Class II medical devices, back to the OEM intended design and are proven to be safe and effective.
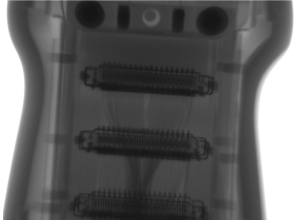
Let’s use an ultrasound probe as an example, and something seemingly as simple as the lens.
To the untrained eye, the lens is just a rubber membrane separating the acoustic array from the patient’s body, but there is much more to it than that. This component is called a lens for a reason:
- It is partially responsible for focusing the acoustic beam from the acoustic stack within the probe
- It protects acoustic matching layer(s), scan head electronics, and the array itself while also electrically isolating the probe from the patient.
- The material itself must have exact dimensions regarding shape and thickness to perform as the OEM intended. If the shape is incorrect by a minute amount, you will lose focus in your image. If the thickness is incorrect, you will affect the amount of energy transmitted to the patient as well as the sensitivity of the probe (especially in Doppler).
- The lens material and any seals must be of a material that is ISO 10993 compliant. 10993 certified materials are certified for their bio-compatibility and bio-stability. Learn more about ISO 10993 here.
Looking at materials in general, besides functioning as the OEM intended, we must also look at biological and chemical compatibility. If a medical device contains materials which may contact patients, the manufacturers should be in compliance with ISO 10993 which is specific to bio-compatibility – in other words, the materials are tested to ensure biological safety. This may include (in the case of probes and coils but extends to other medical devices) lenses, plastics, housings and even adhesives. When performing repairs on these devices, a quality provider will ensure that their materials are also ISO 10993 compliant.
Chemical compatibility is an entirely different matter:
Have you ever looked at the User Manuals for the various OEMs and all the various chemicals that are approved for cleaning? Disapproved? Even some approved chemicals may only be used on certain portions of devices and there are hundreds of probes and coils and hundreds of chemicals in circulation today.
In the case of ultrasound probes, devices are subjected to submersion, harsh chemicals, and in some cases, high heat and vaporized chemicals. If all the materials have not been tested, you will likely get discoloration, degradation and in some cases, the materials will distort or disintegrate. All materials used in repair should be tested to ensure compatibility with OEM recommended cleaners and disinfectants.
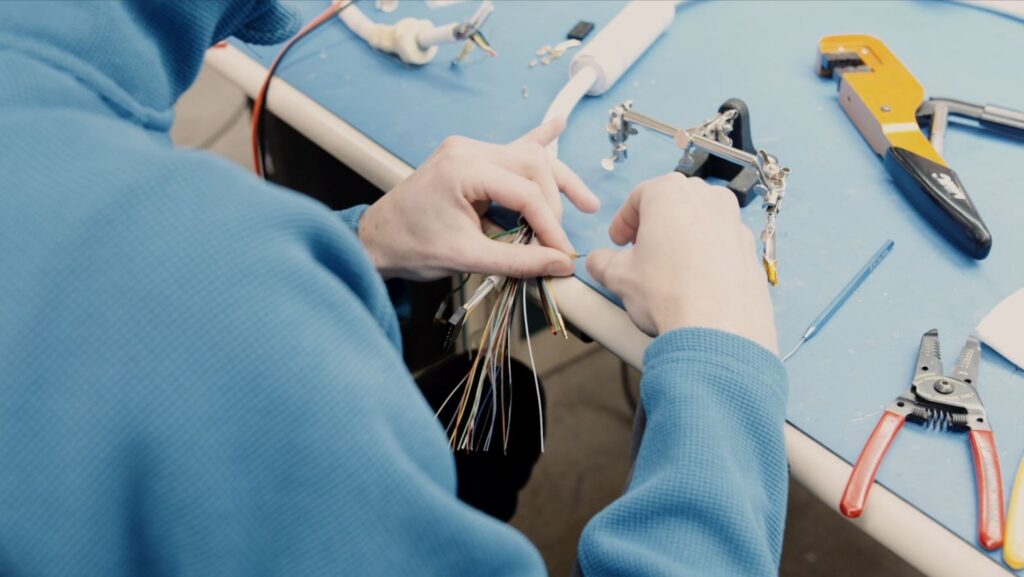
The cable harness in an ultrasound probe or MRI coil is another seemingly simple assembly – just a bunch of wires which transmit and receive, right? Not even close. Different makes and models of probes and coils have specific lengths, diameters, electrical characteristics including impedance, capacitance, reactive capacitance, inductance, stranded or solid center conductor, shielded or unshielded, and even cable sheath colors. These harnesses must be engineered to perform as the OEM intended or you may compromise the performance of the device.
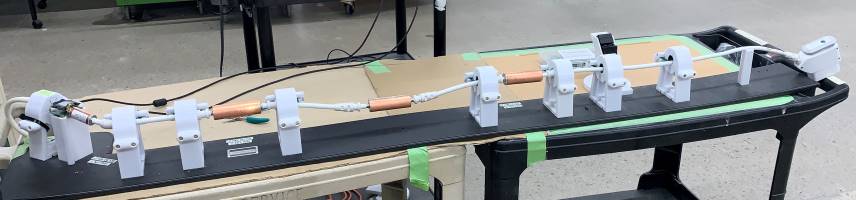
These are just a couple of items as examples in proper MRI coil and ultrasound probe repairs – this list is much longer. When you think about sending out your next repair, check with your repair provider about what may be “inside” to ensure you and your patients get what you should expect and deserve from your repair provider.