Ultrasound transducers are highly complex, Class ll medical devices we see all the time on television, in print ads, and, of course, healthcare facilities. Have you ever given thought to how they are designed, manufactured, tested and repaired? Let’s take a look at the lifecycle of an ultrasound probe.
Innovatus Imaging maintains two facilities dedicated to ultrasound probes: Our FDA registered Center for Design and Manufacturing in Denver, Colorado and our Center of Excellence for Ultrasound Repair in Tulsa, Oklahoma which, together, combine to address the entire lifecycle of transducers including design, engineering, manufacturing and finally restoration to OEM form, fit and function.
Product Design
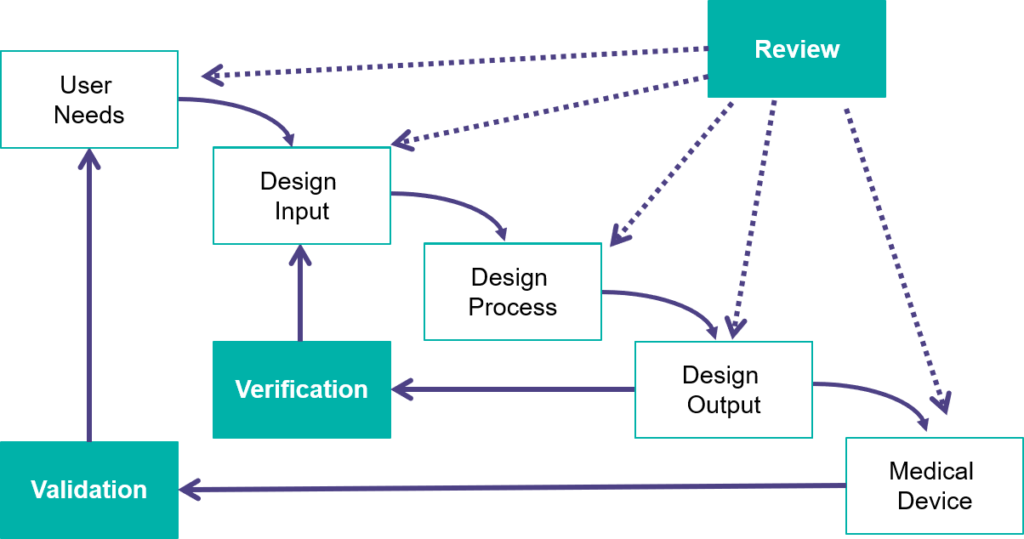
An ultrasound probe, like any other medical device, begins life as a series of user needs, intended use(s) and requirements (or design inputs) and ultimately ends as a finished product, but there is a tremendous amount of work and testing that occurs in between. This includes electrical, mechanical, acoustic and chemical verifications and validations. We need to ensure we are manufacturing a safe and effective product in which output equals input or did we make the transducer correctly? Further, it’s critical that the product meets user needs and intended use(s), or did we make the correct product? Our Denver facility manufactures ultrasound probes from concept to production and must follow FDA regulations every step of the way.
Product Service
Many of the same manufacturing processes and philosophies used in new product development are implemented when we engineer solutions for ultrasound probe restoration. The objective is to return the ultrasound probe to OEM form, fit and function and not significantly change safety, performance, or intended use. Because we are a probe manufacturer, we have all the necessary instruments, knowledge, training, and processes to ensure this outcome as well as to design and manufacture proprietary test and repair fixtures based on the specific needs of various OEM makes and models.
Verification
Does the repaired product fulfill the established TECHNICAL specifications
Validation
Does the repaired product fulfill the required needs of the various users
Let’s look at a component which may appear simple: the transducer cable. There are one-size-fits-all solutions available, but there are many electrical parameters (characteristic impedance, DC resistance, shielding) and physical characteristics (length, diameter, color) which differ from probe to probe and OEM to OEM. Many healthcare technology management professionals don’t realize that OEMs do not share their design specifications with ANY third-party repair provider. Using sophisticated instruments, we benchmark new OEM cables to determine their specifications and then design to those specifications. These cables are then stress tested using customized “torture” devices as well as undergoing electrical and acoustic verification. In fact, we currently fabricate over 80 different cable solutions to ensure OEM-like performance.
Plastics and lens materials may seem simple as well, until you research the dozens of OEM recommended cleaners and disinfectants for the hundreds of probes we restore. All materials used must be not only compatible with recommended cleaners and disinfectants but ISO 10993 compliant (bio-compatible).
Putting It All Together
Once our Design and Manufacturing Center completes and approves a material or process, the details are then transferred to our ISO 13485:2016 registered Ultrasound Repair Center to be implemented into our probe restoration processes. This is continuous, as new devices enter the market and will have different characteristics and designs which we will have to engineer. By being an ultrasound probe OEM, we understand the entire lifecycle of ultrasound probes and, after over 160,000 probe repairs to date, we understand not only how they fail but how to restore them to provide many additional years of patient care.

